✔ 100% Authentic Product
👁️ Currently Viewing 2776
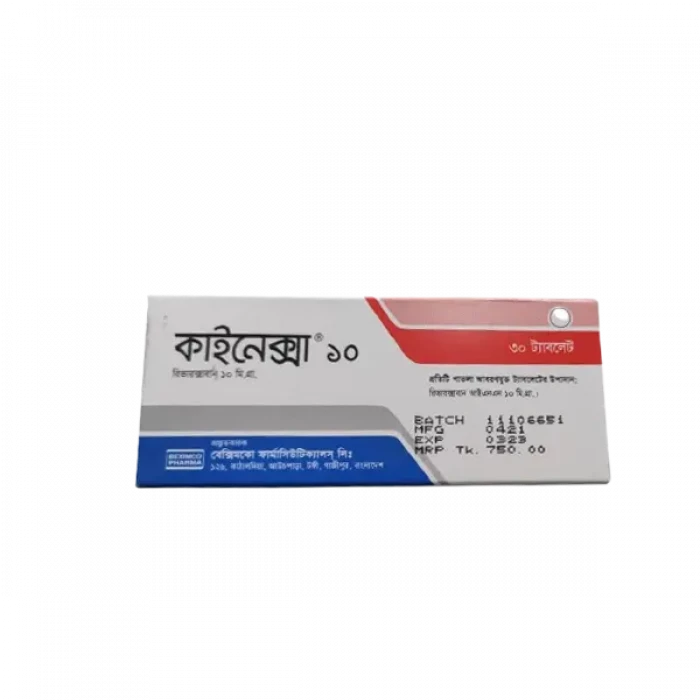
Kinexa 10mg (30pcs Box)
Generic Name: Rivaroxaban 10mg
Company Name: Beximco Pharmaceuticals Ltd.
Discount
Price: ৳ 713
MRP:
৳
750
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Indications
Rivaroxaban 2.5 mg:
For the prevention of atherothrombotic events in adult patients with increased cardiac biomarkers following an Acute Coronary Syndrome (ACS) (Troponin or CK-MB). It is used in combination with aspirin alone, aspirin + Clopidogrel, or Tidopidine.
Rivaroxaban 10-20 mg:
In individuals with nonvalvular atrial fibrillation, to minimize the risk of stroke and systemic embolism.
Deep vein thrombosis (DVT) and pulmonary embolism (PE), as well as the risk of DVT and PE recurrence.
In patients having knee or hip replacement surgery, for the prevention of DVT, which can progress to PE.
Pharmacology
Rivaroxaban is a direct factor Xa inhibitor with high selectivity. Factor Xa inhibition reduces thrombin production by interrupting the intrinsic and extrinsic pathways of the blood coagulation cascade. Rivaroxaban has been shown to have no impact on platelets and does not block thrombin (activated factor II).
Dosage & Administration
Rivaroxaban 2.5 mg:
The recommended dose:5 mg twice daily. Patients should also take a daily dose of 75-100 mg Aspirin or a daily dose of 75-100 mg Aspirin in addition to either a daily dose of 75 mg clopidogrel or a standard daily dose of ticlopidine.
Rivaroxaban 10-20 mg:
Nonvalvular Atrial Fibrillation: For patients with Creatinin Clearance >50 mL/min: 20 mg orally, once daily with the evening meal. For patients with Creatinin Clearance 15-50 ml/min: 15 mg orally, once daily with the evening meal.
Treatment of DVT & PE: 15 mg orally twice daily with food for the first 21 days for the initial treatment of acute DVT or PE. After the initial treatment period, 20 mg orally once daily with food for the remaining treatment.
Prevention in the risk of recurrence of DVT and of PE: 20 mg once daily with food.
Prophylaxis of DVT following Hip replacement surgery: 10 mg once daily for 35 days.
Prophylaxis of DVT following knee replacement surgery: 10 mg once daily for 12 days.
May be taken with or without food.
Interaction
Kinexa 10 exposure and pharmacodynamic effects (factor Xa inhibition and PT prolongation) are increased when used with medicines that are a combination P-gp and CYP3A4 inhibitors (ketoconazole, ritonavir, clarithromycin, erythromycin, fluconazole, diltiazem, verapamil, dronedarone). Rivaroxaban should not be used with a combination P-gp and strong CYP3A4 inducer (e.g., rifampicin, phenytoin, carbamazepine) since it reduces the effectiveness of Rivaroxaban. The use of antiplatelet medicines, heparin, fibrinolytic treatment, and NSAIDs at the same time may increase the risk of bleeding.
Contraindications
It is not recommended for patients who have a known hypersensitivity to Kinexa 10 or any of the product's excipients. It is also not recommended for individuals who are experiencing active pathological bleeding.
Side Effects
Increased risk of bleeding, spinal or epidural hematoma, and increased risk of stroke after cessation in nonvalvular atrial fibrillation are the most prevalent adverse effects of Kinexa 10.
Pregnancy & Lactation
Kinexa 10 is classified as a pregnancy Category C medication. Rivaroxaban has not been studied in pregnant women insufficient or well-controlled trials, and dosage for pregnant women has not been established. Rivaroxaban is not known to be excreted in human milk. Rivaroxaban's safety and effectiveness in nursing mothers have yet to be determined.
Precautions & Warnings
Premature discontinuation of Kinexa 10, in the absence of adequate alternative anticoagulation, increases the risk of thrombotic events. Rivaroxaban increases the risk of bleeding that can be fatal in presence of the following risk factors- bleeding disorders, uncontrolled severe arterial hypertension, gastrointestinal disease (e.g., inflammatory bowel disease, oesophagitis, gastritis, and gastroesophageal reflux disease), vascular retinopathy, bronchiectasis, history of pulmonary bleeding, Signs or symptoms of neurological impairment should be monitored in case of neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture as epidural or spinal hematoma can occur. Rivaroxaban is not recommended in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy.
Storage Conditions
Do not store above 30° C. Keep away from light and out of the reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.